Biomedical ultrasounds
This research theme concerns the development of ultrasound methods for the characterization and diagnostic imaging of biological tissues (bone, blood, tumor), and the ultrasound stimulation of cellular processes for therapeutic purposes. Actions focus on understanding wave-tissue interaction mechanisms and developing inversion methods for building quantitative images, based on analytical, numerical and experimental approaches in vitro and in vivo.
Permanent members : E. Debieu (IE), E. Franceschini (DR), R. Guillermin (IR), P. Lasaygues (IR), F. Legrand (MCF ECM), V. Monteiller (IR), S. Rakotonarivo (MCF AMU), T. Scotti (IR)
Quantitative Ultrasound & Microstructure of Biological Tissues
Our aim is to probe the microstructure of complex biological media (blood and tumors) by ultrasonic backscattering in order to detect pathologies and monitor the effectiveness of treatments. A new ultrasound technique based on structure factor measurement has recently been developed at the LMA to measure the microstructure of dense sheared suspensions of red blood cells (=blood) and dense clusters of cancer cells (=tumors). Microstructure (i.e., the spatial arrangement of cells relative to one another) provides quantitative information on cell properties and interactions. For blood disorders such as sickle cell anemia, our current studies are evaluating the effectiveness of this quantitative ultrasound technique for detecting altered deformability and aggregation of red blood cells (ANR HEMO 2022-2026). For cancerous tumors, our studies focus on understanding ultrasound scattering by isolated cells or cell clusters during a cell death process, with a view to monitoring anticancer therapy (ITMO NIFUS 2022-25).

Example of histological sections of HT29 cancer cell biofantoms untreated and treated with a chemotherapeutic agent. Comparison of ultrasound intensities measured and predicted by the polydisperse structure factor model in the 10-32 MHz frequency band.
Nonlinear ultrasound tomography: application to musculoskeletal ultrasonography in children
This theme concerns the development of non-linear ultrasound tomography. In contrast to conventional linear approaches, image reconstruction is envisaged here by constructing a cost function that depends on the difference between the measured and simulated fields; identification is achieved by searching for its minima. The modeling of the direct problem is based on a variational formulation of the elastodynamic equations, solved in 3D using the spectral element method (SPECFEM3D open-source software). The inversion algorithm is based on iterative descent methods (quasi-Newton method) and Full Waveform Inversion (FWI). The target application is musculoskeletal ultrasonography in children. The non-linear version of ultrasonic tomography enables us to take into account complex propagation media made up of soft tissue, muscle and long bone, with high acoustic impedance contrasts.
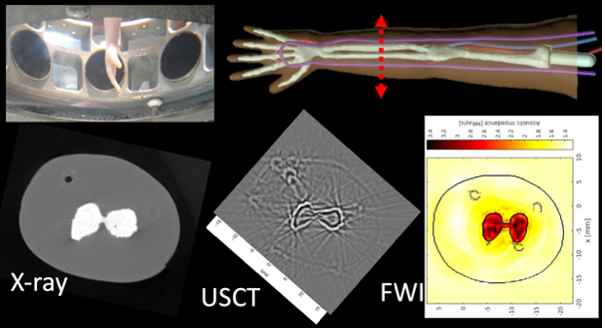
X-ray microtomography imaging (Fédération Fabri de Peiresc), and linear and non-linear ultrasound tomography of an artificial phantom of a child's arm (True PhantomTM, CA) containing the humerus, radius and ulna, humeral, radial and ulnar veins, embedded in homogeneous adipose tissue (coll. LMA- Institut Clément Ader, Tarbes). (Top left) LMA 8-channel circular diffraction antenna.
Ultrasonic stimulation of cellular processes
Ultrasound is a promising tool for non-invasively activating or modulating remote cellular processes in deep organs. The two targeted cellular processes are bone remodeling (ANR INVICTUS 2023-2027) and neurostimulation of sensory neurons (ANR HEAR-US 2020-2024). Our objectives are (1) to identify the optimal ultrasound parameters required to trigger biological responses in a controlled and reproducible way, and (2) to understand the physical mechanisms involved in these processes. Experimental devices are being developed to measure the effects of ultrasound on bone cells or neurons in culture, using SEM or calcium imaging. Numerical simulations and measurement campaigns are conducted to assess which ultrasound-induced phenomenon(s) (acoustic radiation force, acoustic streaming, temperature change) are responsible for activating these cellular processes in our in vitro and in vivo experiments.

(a) Calcium imaging of isolated sensory neurons, 2 s. before the start of ultrasound stimulation, then 2 s. and 40 s. afterwards (coll. LMA-IBDM). (b) Multi-scale numerical modeling of acoustic streaming in a 2D section of a bone scaffold (coll. IRPHé-LMA).